Abstract
Introduction: Intensive chemotherapy with 7+3 or CPX-351 remains standard of care for fit adults with newly diagnosed AML. Nearly 50% achieve a remission after one induction cycle, and up to an extra 20% can achieve remission after a second intensive cycle. However, intensive re-induction increases morbidity through significant prolongation of cytopenias. Furthermore, certain patients (pts) may be less likely to respond to intensive re-induction, namely those with adverse risk disease. Recently, with effective non-intensive options, such as venetoclax (Ven) combinations, or IDH inhibitors, we offered low intensity re-induction to select pts with persistent disease after initial intensive therapy as a potential means of reducing morbidity, and potentially improving chance for response. This study describes the characteristics and clinical outcomes of pts with residual disease after intensive induction, treated with either Ven or IDH inhibitor re-induction.
Methods: A chart review of ND-AML pts treated between 1/2017 and 2/2022 was performed. We identified pts who either received 7+3 or CPX-351 for initial induction. We then selected pts who had a nadir BM biopsy demonstrating persistent AML (>5% blasts), who then received either a Ven combination or an IDH inhibitor as next treatment. To characterize pt burden, we collected hospitalization duration, PRBC and platelet transfusion burden over 30 days after re-induction, neutropenic fever rates, ICU admission rates, and death within 30 days. We also collected clinical outcomes including CRc after 1 cycle of therapy, best response, proportion of pts proceeding to allogeneic HCT (alloHCT), and leukemia free survival (LFS).
Results: A total of 15 pts were identified. Median age of pts was 61 years (22-67), 5 (33%) pts had ELN intermediate risk disease, 10 (67%) pts had ELN adverse risk disease; 5 (33%) pts had mutations in IDH1/2, and 4 (27%) pts had mutations in TP53. Most pts, 11 (73%), received initial induction with 7+3 chemotherapy; on post induction nadir marrow, a median 34% (10-90%) blasts were observed.
For re-induction, 11 (73%) pts received a Ven combination (either with decitabine or azacitidine), and 4 (27%) received an IDH1 inhibitor (with azacitidine in 2 pts). Most common reason documented for re-induction choice was adverse risk disease, followed by presence of IDH mutation, followed by loss of fitness for additional intensive therapy.
Median hospitalization duration from induction day 1 was 27 days (20-41); median time in hospital after re-induction was initiated was 6 days (0-27). After initiation of re-induction therapy, over the next 30 days, median number of PRBC transfusions administered to these pts was 1 (0-8), median number of platelet transfusions was 2 (0-17). Overall, 2 (13%) pts were re-hospitalized for neutropenic fever, including 1 (8%) pt who was admitted to the ICU and subsequently died (Table 1).
In terms of response, 9 (60%) pts achieved a CRc after one cycle of low-intensity therapy. This includes 6 pts who received a Ven combination, and 3 patients who received ivosidenib (with or without azacitidine). Subsequently, 8 (73%) pts on a Ven combination achieved a CRc as best response, and 4 (100%) pts achieved CRc utilizing ivosidenib (Table 1).
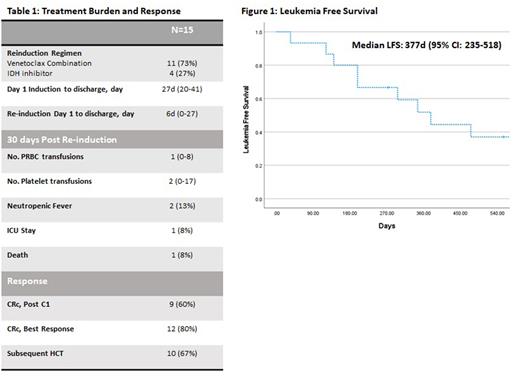
Ten (67%) pts proceeded to alloHCT. Overall, median leukemia free survival was 377 days (95% CI 235-518), and 7 (47%) pts remain alive at last follow-up (Figure 1).
Conclusion: Among pts with residual disease after initial intensive induction, switching to a Ven combination or IDH inhibitor potentially reduces pt burden through shorter hospital stays, and lower burden of neutropenic fevers and transfusions. Furthermore, this strategy has promising efficacy, and serves as a successful bridge to alloHCT. A control cohort of pts receiving intensive re-induction is being composed from pts treated at our institution and will add further context.
Disclosures
Abedin:Incyte: Research Funding; AbbVie: Honoraria; Amgen: Honoraria; Stemline: Honoraria; AltruBio Inc.: Research Funding; Helsinn Healthcare: Research Funding; Pfizer: Research Funding; Actinium Pharmaceuticals: Research Funding. Runaas:Servier: Honoraria. Michaelis:Jazz Pharmaceuticals: Other: Funding for Research Study to my Institution; Abbvie: Consultancy, Other: Consulting, Advisory Board Meeting; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees, Other: Consulting, Advisory Board Meeting; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees, Other: Consulting, Advisory Board Meeting; Incyte Corporation: Consultancy. Atallah:Takeda: Research Funding; Abbvie: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; BMS: Consultancy, Speakers Bureau; Blueprint: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal